Our Facilities
Office
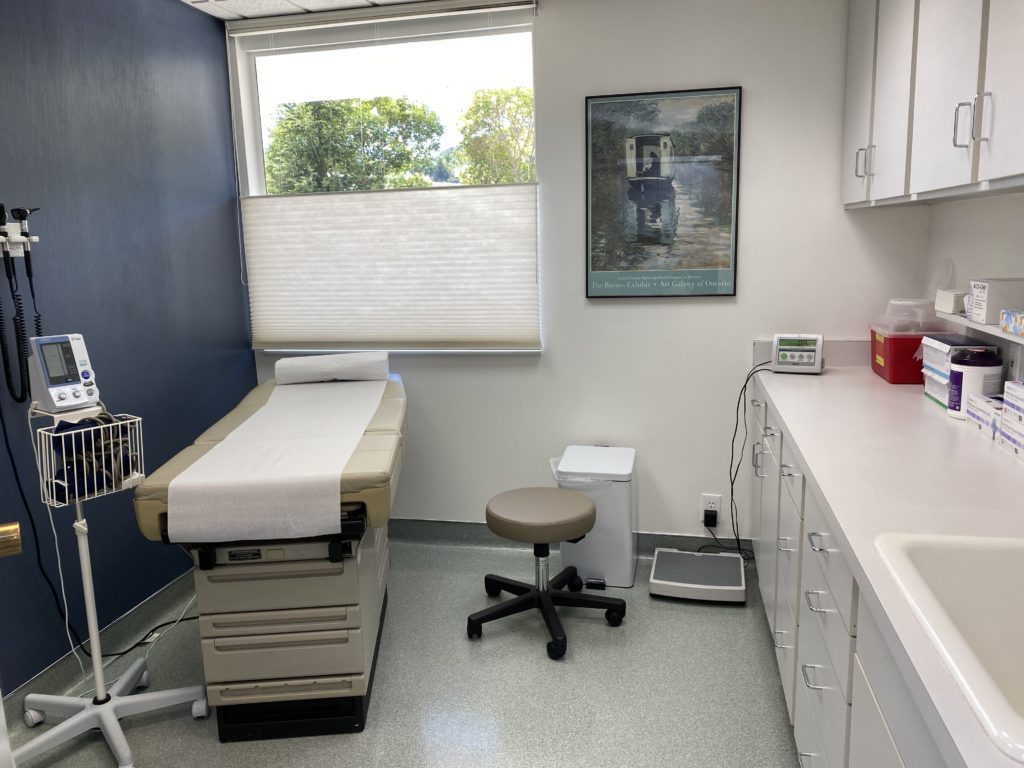
Site has a dedicated office space (870+ sq ft) that has its own entrance and waiting room, and includes 2 fully equipped private patient exam rooms, where clinical trial visits are conducted. There is individual desk space for each study coordinator with phones, computers, and storage space. Dedicated locked drug storage space is available for Ambient and Refrigerated Investigational Product (IP).
There is easy access to all office supplies and equipment in common work areas (printers, fax machine, copier, mail supplies). Space is allocated for research staff only and is not accessed by non-research staff. Two individual work areas are also designated for Sponsor Site Monitors. Additional off-site storage for completed study materials is also maintained by site.
We have experience with the most recent Electronic Data Capture systems including Oracle In-Form, Rave, IBM (Zelta), Veeva, and Medpace. Also have significant experience using equipment for clinical trials such as Dexcom CGM, ERT and Clario electronic diaries, IQVIA ABPM ambulatory blood monitoring and ECG systems.
Laboratory
- Site has a designated lab to process clinical trial lab samples, certified with a CLIA waiver (CLIA ID#05D0594923).
- Lab contains both a standard and refrigerated centrifuge, as well as a refrigerator and -30 freezer.
- Staff members are also IATA certified to ship PK and frozen lab samples.
- Site affiliated with local organizations providing Bone Density Scans with Body Composition